Heart failure (HF) is a pressing and illusive public health epidemic. Nearly 50% of all HF cases involve HFpEF — to which there is no defined therapy (1). As such, refining prediction and treatment of HF is a crucial step in alleviating high morbidity and mortality (2). The development of novel clinical tools and therapies that assist in the diagnosis and management of HF, such as the use of biomarkers, are essential to improving patient outcomes.
Beyond specified patient management, developing our understanding of biomarkers has the potential to stimulate new avenues for research and development, and uncover new sites for therapy (3). Briefly, biomarkers are circulating substances in the blood which help physicians to better understand the unique biochemical underpinnings of pathophysiology specific to each patient (4). This is key as patients could have an identical clinical appearance, while having wildly different cardiac structure and function. Biomarkers will allow physicians to identify HF patients — particularly those with HFpEF, on a more objective level (5). Further, biomarkers themselves may be risk factors and therefore potential targets of therapy (6).
Individual biomarkers are now commonplace in clinical practice — notably, BNP and NT-pro-BNP are class 1a for diagnosis and prognosis of HF (7). While there are many advocating for the utility of biomarkers (8) others posit that the reliability and clinical utility of many established biomarkers remains uncertain (9). Established biomarkers within the HF field have failed to improve therapy results and are inaccurate in assessing the complex and varied pathophysiological processes which underpin HF (9, 10). Therefore, ongoing research in new and emerging biomarkers alike should adhere to ‘rigorous evaluation’ to ensure they are valuable to physicians and patients (11).
Beyond standalone biomarker investigations, there are numerous studies seeking to combine multiple biomarkers to one integrated panel. This multimarker approach seeks to improve diagnosis, monitoring, and therapy in HF. Individual biomarkers are integrated into one derivative score, from which an objective classification of a patient is given. This approach seeks to provide a comprehensive overview of the specific biochemical makeup of individual HF patients, as well as insight into the intricate pathophysiological underpinnings of HF (ibid.).
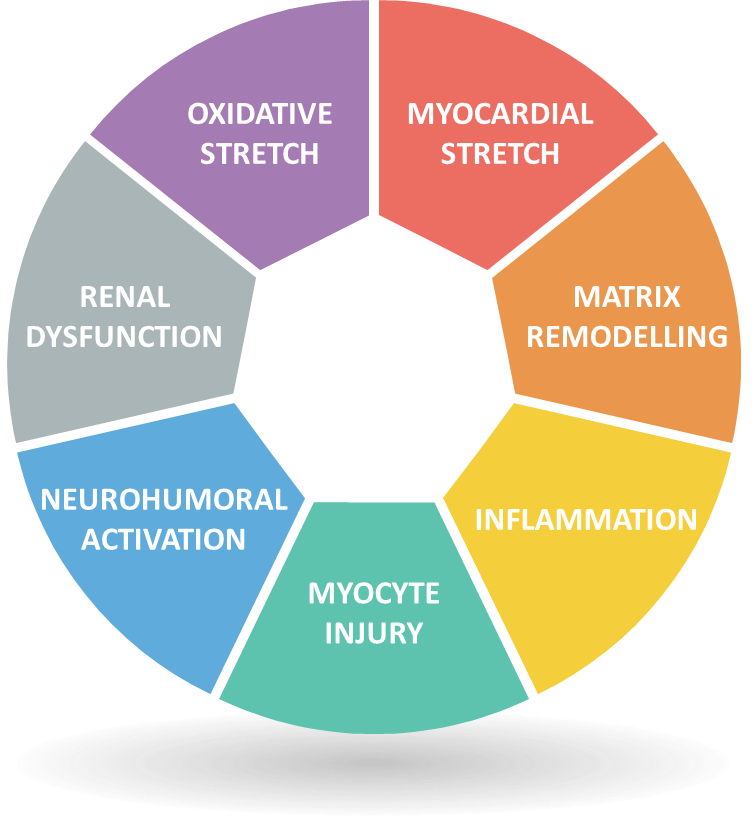
Figure 1: Seven major classes of Biomarker contributing to the Biomarker profile in HF (Adapted from Heart Failure by E Braunwald, 2013, JACC: Heart Failure, 1(1):1-20)
‘Braunwald’s star’ [Fig 1] can be used as a framework to which combinations of biomarkers can be mapped across the varied and interconnected biological pathways relevant to HF prognosis. For example, a multimarker approach employed by Bayes-Genis et al. (13) based on NT-pro-BNP to indicate myocardial stretch and neurohumoral activation, hsTnT to indicate myocyte injury and ST2 to indicate matrix remodeling and inflammation. This approach showed an increase in the identification of high-risk patients for recurrent hospitalizations. Another combination, utilized by Bauer et al. — hsTnT, NT-pro-BNP and IGF-BP7 — increased prognostic value and identification of patients with a high-risk of mortality and rehospitalization (14). While it is established that multimarker approaches yield improved prediction of adverse events (15), identifying an optimal panel of biomarkers for assessing HF¬ remains a pivotal step in the pursuit of advanced diagnosis, prognosis and specific therapy (10).
Beyond the realm of HF, this framework could be an exciting and fruitful approach to research and treatment in a variety of therapeutic areas. Biomarkers are an essential tool in numerous other medical disciplines and the application of a multimarker approach may yield novel treatment options and improved patient outcomes. Naturally, these multimarker approaches will require an adept and nuanced communication initiative to ensure that the approach is applied in a functional and effective clinical manner. When such research needs to be translated into clinical settings, The Corpus hopes to provide such a space for the intimate and detailed discussion required to connect and update physicians on novel development in the field of biomarkers.
If you would like to find out more about how you can support programmes in this therapy area, or any other therapy area, please contact us at communications@the-corpus.com.
References
1 — Oktay, A.A., Rich, J.D. and Shah, S.J., 2013. Curr heart fail rep, 10(4), pp.401-410.
2 — Velagaleti, et al., 2010. Circ, 122(17), pp.1700-1706.
3 — Gaggin, H.K. and Januzzi Jr, J.L., 2013. Biochimica et Biophysica Acta (BBA)-Mol Basis of Disease, 1832(12), pp.2442-2450.
4 — Strimbu, K. and Tavel, J.A., 2010. Curr Opinion in HIV and AIDS, 5(6), p.463.
5 — Shere, A., Eletta, O. and Goyal, H., 2017. J Lab Precis Med, 2(99), pp.1-11.
6 — Kalogeropoulos, A.P., Georgiopoulou, V.V. and Butler, J., 2012. Prog in cardiovasc diseases, 55(1), pp.3-13.
7 — Ponikowski P.,, et al., 2016. Eur heart J, 37(27), pp.2129-2200.
8 — Nadar, S.K. and Shaikh, M.M., 2019. Cardiac fail rev, 5(1), p.50.
9 — Spoletini, I., et al., 2019. Eur Heart J Supp, 21 (Supplement_M), pp.M5-M8.
10 — Topf, A., et al., 2020. Frontiers in Cardiovasc Med, 7.
11 — Januzzi, J.L. and van Kimmenade, R.R., 2014. J Am Coll Cardio
12 — Braunwald, E., “Heart failure.” JACC. Heart failure vol. 1,1 (2013): 1-20.
13 — Bayes-Genis A., et al., 2016. Frontiers in cardiovascular medicine, 3, p.37.
14 — Bauer S., et al. (2020). J ClinChem Lab Med. 3:145.
15 — Ky, B., et al., 2012. Circulation: Heart Failure, 5(2), pp.183-190.